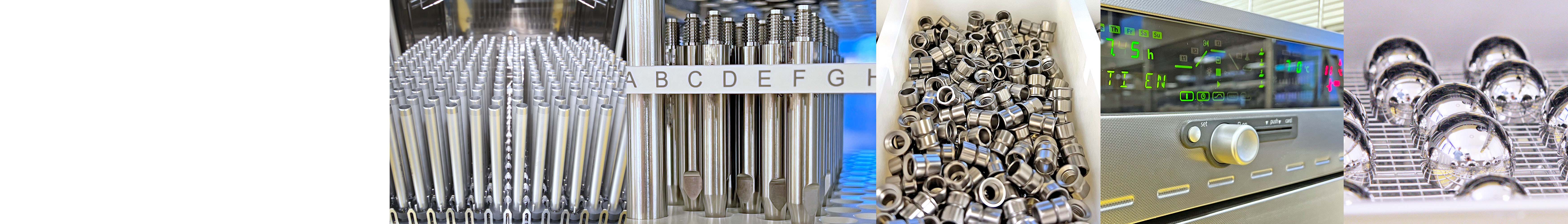
Our engineering, natural science and microbiology teams, skilled workers and those with specialist knowledge bring everything together - sustainably, safely and efficiently!
Only qualified processes and equipment are used to handle products for customers. It goes without saying that these processes are carried out under clean room conditions.
The requirements for the cleanliness of medical products have steadily increased in recent years and form a safe starting point for the further process steps towards a sterile product.
Cleaning downstream of manufacturing can reduce microbiological contamination, known as bioburden, removes chemical contaminants from the manufacturing process and particulate contamination from general manufacturing, transport or storage processes.
Modern and innovative manufacturing processes and methods such as 3D printed implants with complex geometries and structures require equally complex cleaning processes.
Together with our customers, we define a cleaning process that is optimised for the product. Considering standards-related specifications as well as the implementation of cleaning validations is an integral part of this.
In order to be able to offer the optimal cleaning process, Eurofins Steripac GmbH has extensive cleaning facilities:
- Vacuum extraction cleaning technology
- Cleaning and disinfection machines
- Ultrasonic cleaning
- Vacuum drying
- Drying cabinets
- Drying with laminar flow
- Reverse osmosis system for water treatment
There are multi-part products whose components must be partially or fully cleaned. In such a case, assembly at Eurofins Steripac GmbH under clean room conditions following the cleaning process is the obvious choice.
The assembly process is determined in consultation with the customer and may also include subsequent functional or quality tests on the product.
If assembly equipment is necessary for this, it can be provided by the customer or new equipment developed by us together with the customer.
Our employees have years of experience in carrying out assembly steps and are intensively trained and familiarised with new processes.
The packaging is the first thing your customer sees when coming into contact with your product. It must be easy to open and to remove the product according to the function.
At Eurofins Steripac GmbH, we understand that packaging is an integral part of a medical device with key importance for both manufacturer and user.
It serves as a sterile barrier system and fulfils other elementary functions: the packaging ensures that your product is ready for use for the specified shelf life.
Together with the customer, we define the requirements for the packaging system and develop an optimal and product-related solution.
In addition to the various packaging solutions for sterile protection or outer packaging through to the cardboard packaging, we also take into account the requirements for the relevant labelling obligations.
We offer the following types of packaging:
- Four-edge sealed bags
- Soft blister packs
- Hard blister packs
- Individual sterile barriers
Different materials are used, e.g.:
- GAG-PET / G-PET /
- PE or PA composite films
- Coated and uncoated Tyvek
- Aluminium composite films
- Coated and uncoated medical grade paper
What exactly is the 3dpac?
The 3dpac is a blister system developed by steripac for optimal position fixing of sensitive products within the sterile barrier.
How does the 3dpac work?
The 3dpac comprises three components:
- Outer sterile barrier (secondary blister)
- Inner sterile barrier (primary blister)
- Fixing inlay
Your medical device is at the centre of the 3dpac. It is embedded within a thermally custom-shaped fixing inlay in the primary blister in a stable position. The fixing inlay is produced thermally during the packaging process. The moulding for this is the product already within the blister. That is why the inlay always encloses the product like a second skin and provides uncompromising safety.
Products can have different geometries and sizes in each individual packaging process. The fixing inlay is always individually thermally adapted to the product in question.
When removing the product, the user first removes the fixing inlay from the packaging. The actual medical device is easily accessible and intact within the primary blister.
For which products in particular is the 3dpac suitable?
For all highly sensitive medical products! The fixing inlay envelops the product in a tailor-made way, thus enabling movement- and abrasion-free storage in the blister.
The innovative blister design shows its particular strengths in products with a large number of variants in terms of sizes and geometries, as well as relatively small batch sizes
Examples of products with a large number of variants:
Generatively manufactured implants
- Spinal implants
- Screws
- Plates
Sterilisation is the final process for a sterile product. It comes at the end of the production chain and ensures that your product is safe and ready to use over the long term.
The optimum process in each case is selected depending on the product requirement and the product and packaging materials used.
The most common sterilisation methods for medical products are:
- Gamma irradiation
- Sterilisation using ethylene oxide
- Steam sterilisation
- Electron-beam
Our long-standing partner companies who carry out the sterilisation work exclusively on qualified equipment. The processes are validated and documented on a product-related basis in accordance with the relevant standard.
For the processes available at steripac, we offer customised documentation that complies with the guidelines. These include in particular:
Cleaning validation
- Microbiological burden
- Chemical residues
- Particle investigation
- Cytotoxicity
- Endotoxins
- Biocompatibility
Packaging validation
- Seal strength
- Leakage test
- Colour test
- Microbial barrier effectiveness depending on humidity
- Transport simulation tests
- Mechanical load tests
- Accelerated ageing
- Real-time ageing
Sterilisation validation
- e.g. Gamma DIN EN ISO 11137
- e.g. EtO DIN EN ISO 11135
All validations and the associated tests are carried out and documented in accordance with the applicable standard.
The necessary scope may vary and is determined together with the customer.
We always look forward to welcoming you to the important trade fairs and presenting our products and services in person.
We are currently planning the next dates and will inform you here as soon as we have any news.
_